The First Covid-19 Vaccine Doses Are Already Flying
Contributors are not employed, compensated or governed by TDM, opinions and statements are from the contributor directly
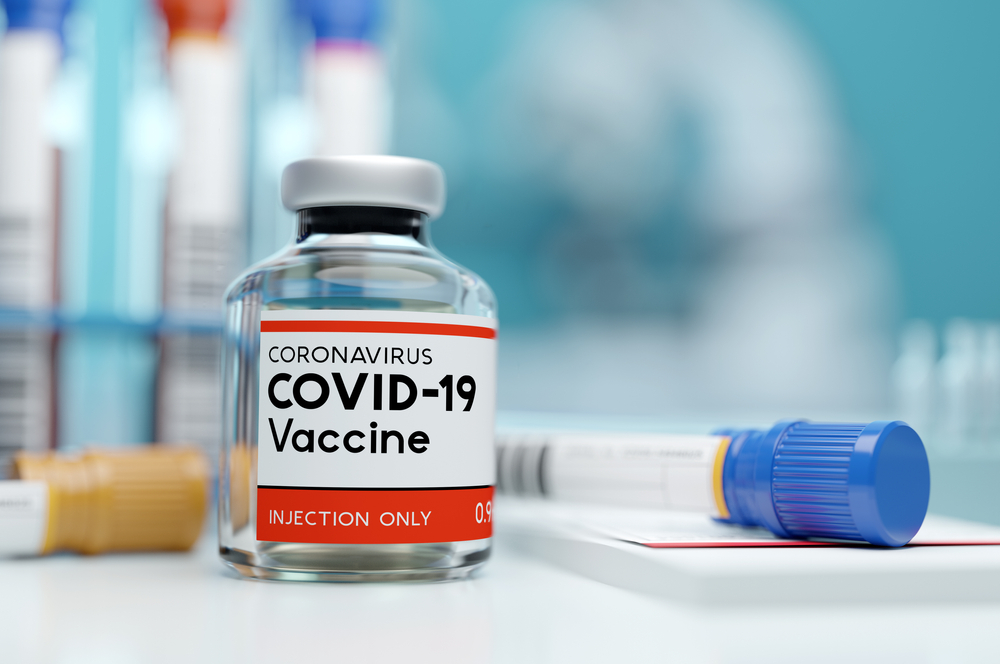
There are a few questions on every traveller’s mind, but two big ones. When will the country I want to visit be open to me? And, when will covid-19 vaccines be making their way to the public? If air cargo is any indication, perhaps the answer to both questions is sooner than you think.
ABC News in the US 3 hours ago reported that the first Pfizer Covid-19 vaccine is on the move to the US. United Airlines has already flown a charter cargo plane from Brussels to Chicago with the first shipment of the vaccine on-board and it will be distributed if and when the vaccine is approved by the FDA (Food and Drug Administration).
The US has over 100 million doses of the Pfizer x BioNTech vaccine on order, with options for multiples more.
United Airlines has not confirmed any details but said that it will support the vaccine distribution on a global scale.
The FAA (Federal Aviation Administration) has granted United Airlines a special permit to carry 6800 Kilo’s of dry ice, which is 5 times more than what is usually permitted, in order to keep the doses of the vaccine cold.
The Pfizer x BioNTech vaccine requires cold storage, which makes logistics a greater challenge than usual, but the extremely high effective rate in stopping covid-19 cases makes it incredibly sought after, nonetheless.
Meantime the U.K. is poised to become the first country to approve Pfizer Inc. and BioNTech SE’s Covid-19 vaccine, ahead of a long line of countries waiting for protection from the coronavirus.
Clearance is possible as soon as early next week, according to a person familiar with the situation, who asked not to be identified because the process is confidential.
The U.K. had long signalled it would move fast on any promising vaccine candidate. Russia and China have cleared vaccines for general use, but they are unlikely to be adopted in the U.S. and Europe.
British doctors were put on standby for a possible rollout before Christmas. The government invoked a special rule allowing the U.K. drug regulator to bypass its European Union counterpart as the country prepares for the Brexit transition period to conclude at the end of this year. And the U.K.’s Medicines and Healthcare Products Regulatory Agency started its own accelerated review.
Pfizer and BioNTech already scaled up distribution centres in Germany and across the USA, and AstraZeneca is doing the same in key markets with its ‘Oxford’ vaccine.
Airlines are said to secretly, or not so secretly now, already be moving vaccine doses from the various vaccine candidates into key global positions, so that once emergency approvals are granted in any given country, limited stockpiles will already exist, or at least the facilities to create them.
It is not going to happen overnight, but many in the pharmaceutical and government world on timing is being downplayed to temper expectations in case any unforeseeable hiccups arise. In a year rife with bad news, it makes sense.
Early vaccine doses for healthcare professionals and the most at-risk groups are said to potentially begin within weeks, or a period shorter than two months in many countries, including the USA and UK. Widespread dose availability may not be seen until mid, or even late 2021, but don’t be surprised if timetables are brought up if emergency approvals are indeed granted.
Even if you don’t immediately receive a dose, the world, and travel with it, will still slowly be returning to normal, as other people do.

Comments are closed.